Description
Type
Color
BET Surface area (m 2 / g)
Pore diameter (nm)
Pore volume (cm 3 / g)
Synthesis method
Powder
Blackish brown
۱۷۰٫۶۵
۸٫۰۷
۰٫۳۴۴
Hydrothermal
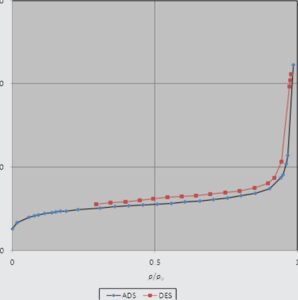
The N adsorption-desorption isotherms and pore distribution analysis for sample shows that the average pore diameter of MnO is 1.25 nm that indicates its microstructure pores
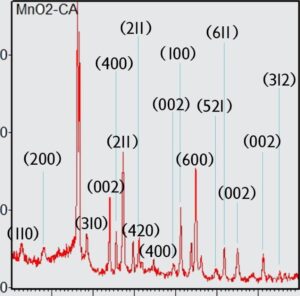
.The results of electron diffraction confirm the presence of alpha-manganese and activated carbon phases
.The characteristic peak at 2-theta 26.8 indicates activated carbon as the catalyst support
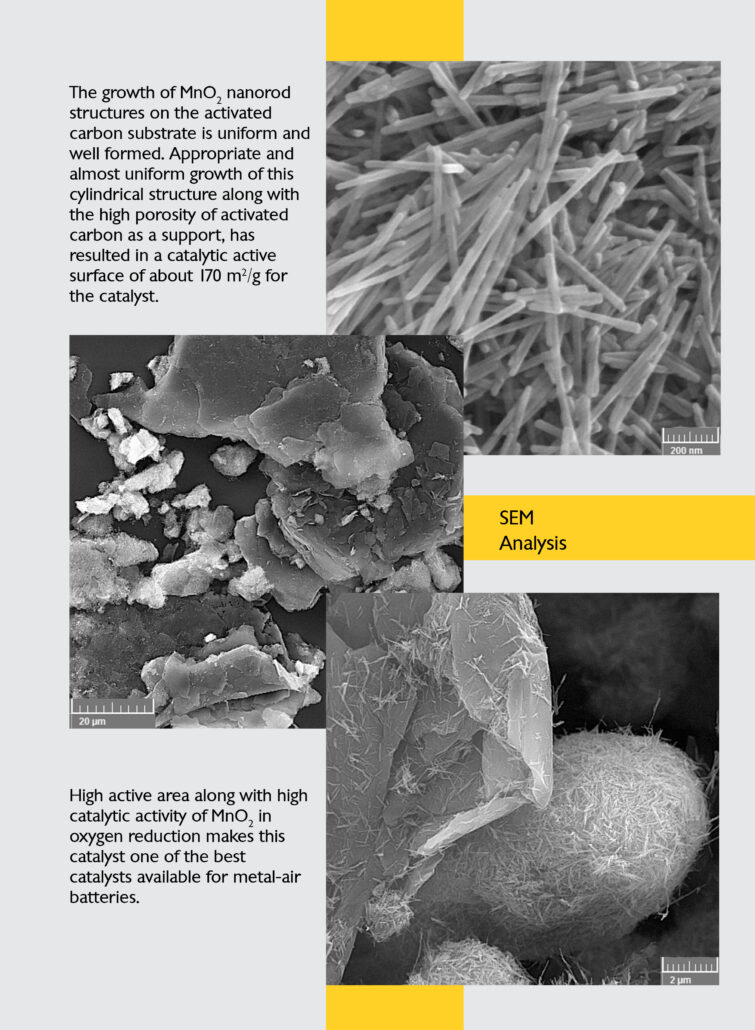
The growth of Mn02 nanorod structures on the activated carbon substrate is uniform and well formed. Appropriate and almost uniform growth of this cylindrical structure along with the high porosity of activated carbon as a support, has resulted in a catalytic active surface of about 170 m2/g for the catalyst
High active area along with catalytic activity of Mn02 in oxygen reduction makes this catalyst one of the best catalysts available for metal-air batteries
Performance comparision of catalysts
Comparison ofthe performance of different catalysts
Oxygen reduction reaction (ORR)
kOH 0. 1 M. electrolyte
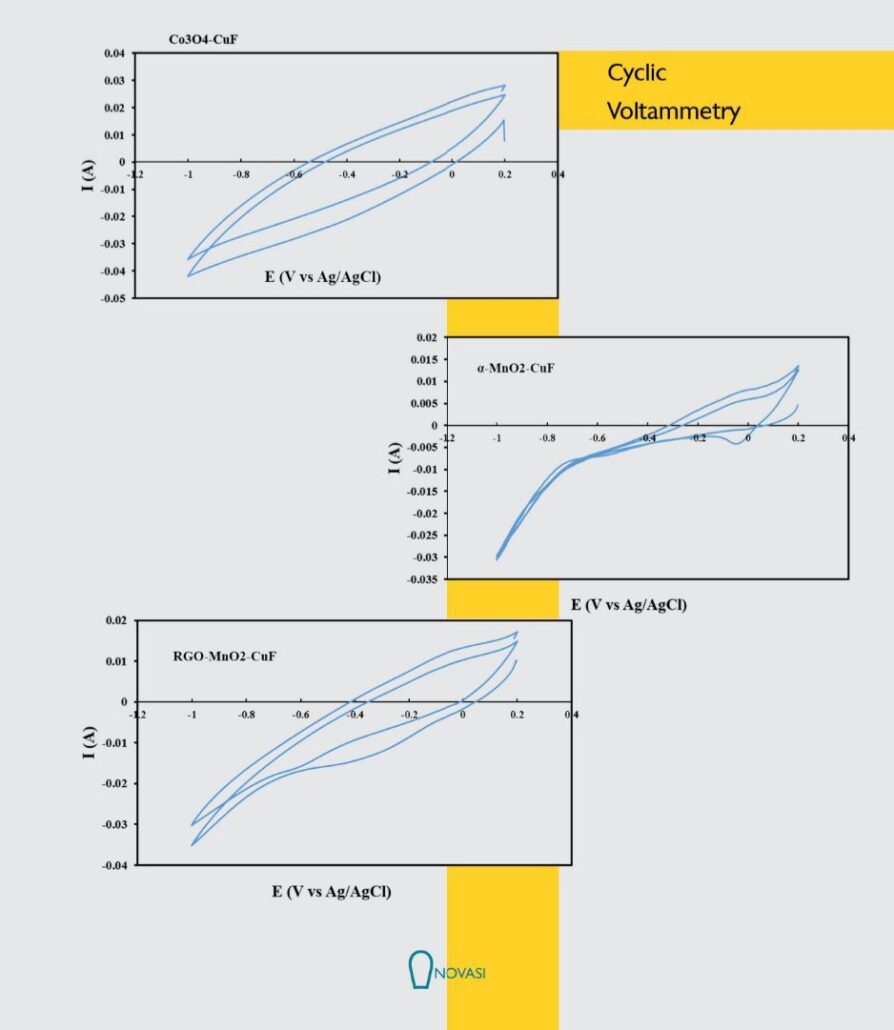
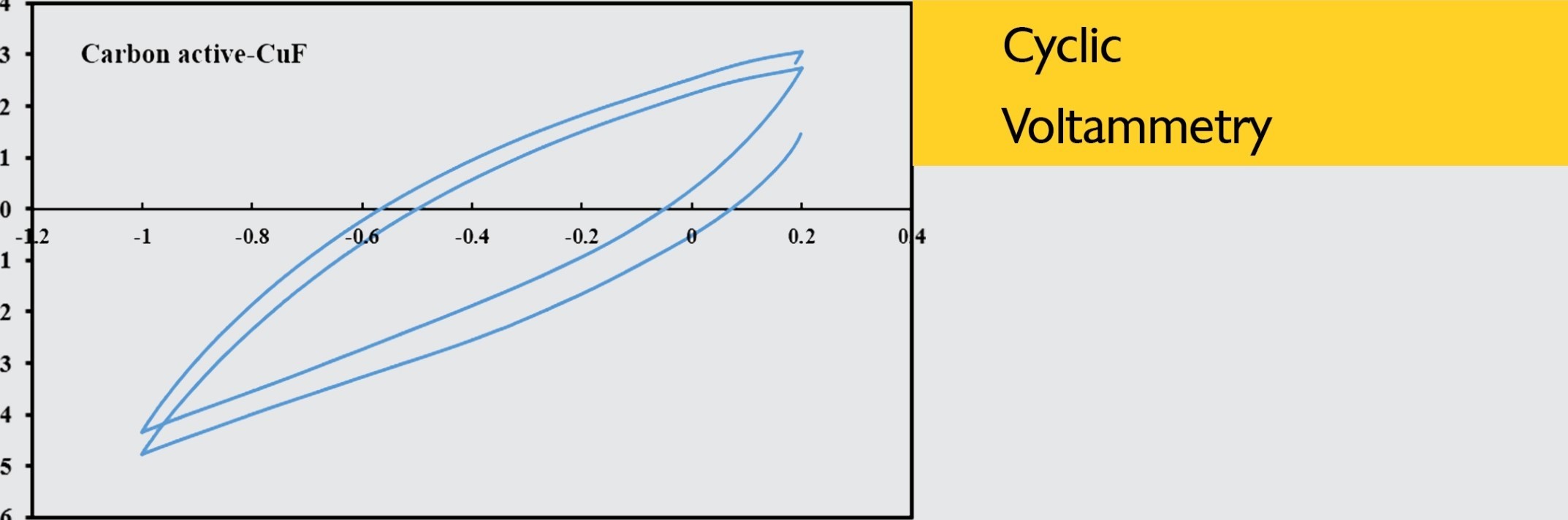
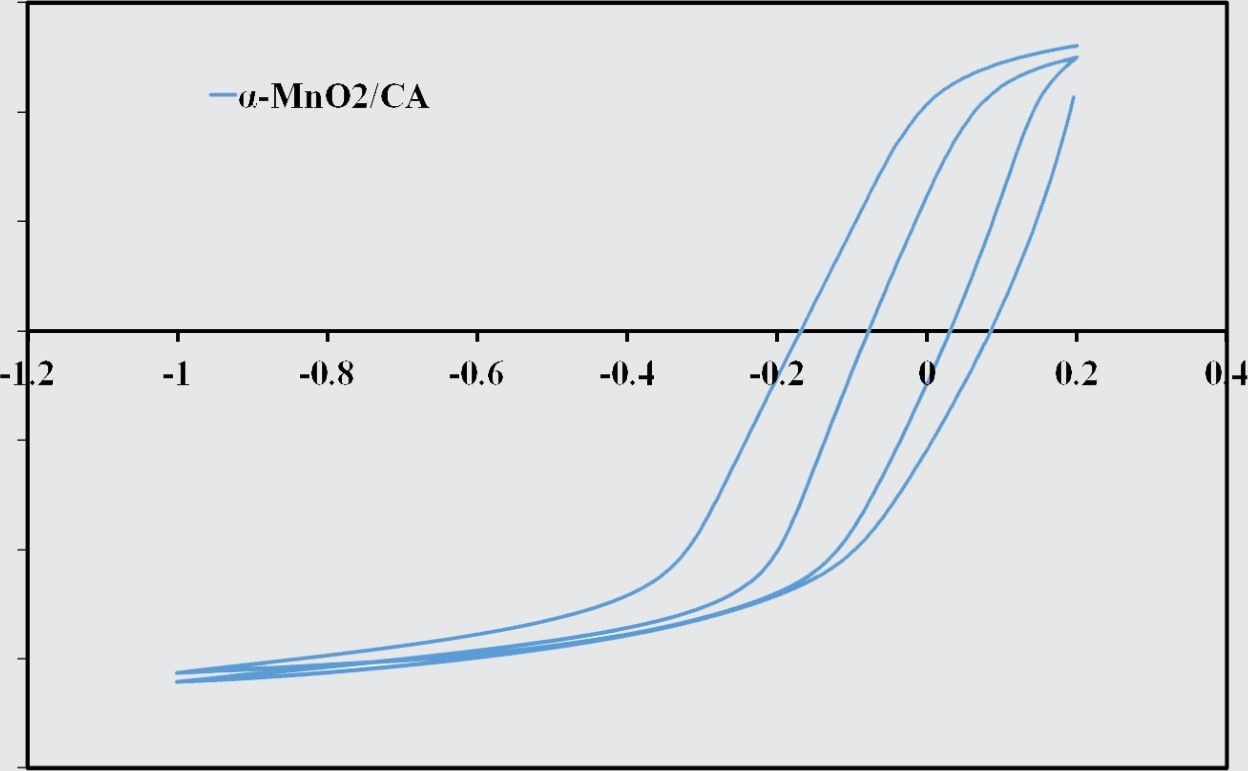
To evaluate the electrocatalytic activity of different catalyst towards oxygen reduction reaction, the cyclic voltammograms (CVs) of five catalysts of Mn02, MnoJCA, CoA-MnoJCA, Mno /RGO and activated carbon were recorded in the presence (O -saturated) of oxygen in 0. I M KOH electrolyte solution at scan rate of 20 mV/s. As can be seen in the cyclic voltammograms (CVs), on the surface of bare carbon cloth electrode in N2-saturated KOH solution, no noticeable oxygen reduction peak is observed while a weak reduction wave is appeared in the 02-purged KOH electrolyte which could be ascribed to the catalytic role of carbon-based substrates for ORR. Among these five catalysts, the RGO a-MnO catalyst shows the most obvious cathodic peak for oxygen reduction, which is in the potential range of 0.2 to -0.6 V
NOVASI