Description
Type
Color
BET Surface area (m 2 / g) Pore diameter (nm)
Pore volume (cm 3 / g)
Synthesis method
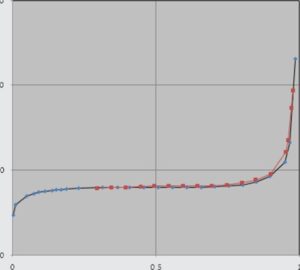
Adsorption / desorption isotherm
Powder
Black
۱۵۰٫۳
۴٫۷۵
۵٫۱۷۸
Hydrothermal
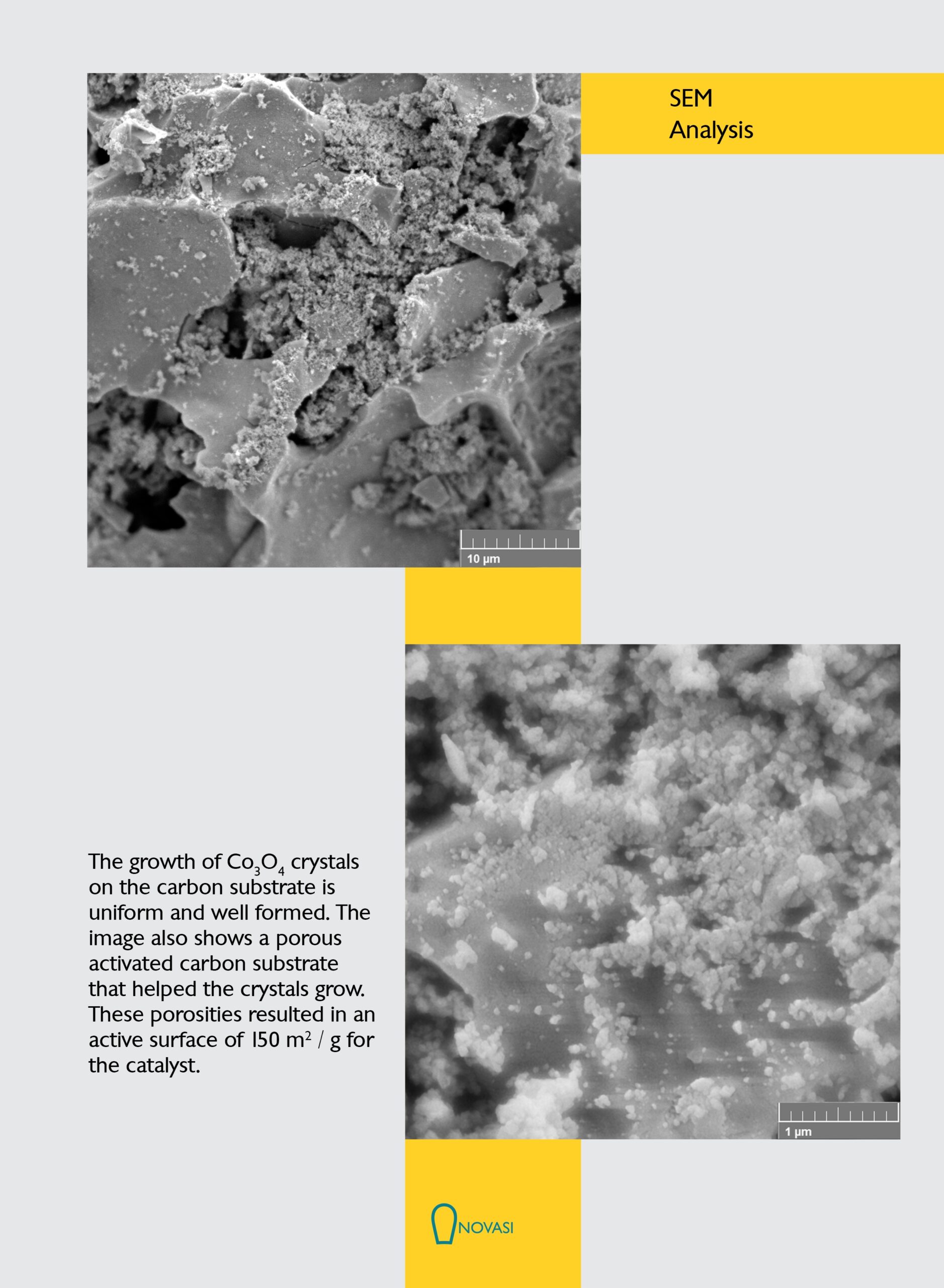
.The BJH diagram of shows that there are two types of meso and macro pores in the structure
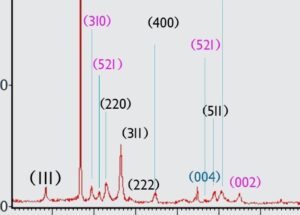
Spectrum analysis shows that the phases in the sample are a combination of a-Mn02, Mn O and C0304 phases. The presence of a small amount of Mn203 phase is probably due to calcination of the sample at 300 0 C
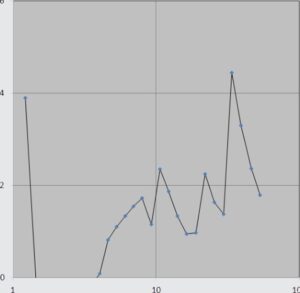
Performance comparision of catalysts
Comparison ofthe performance of different catalysts
Oxygen reduction reaction (ORR)
kOH 0. 1 M. electrolyte
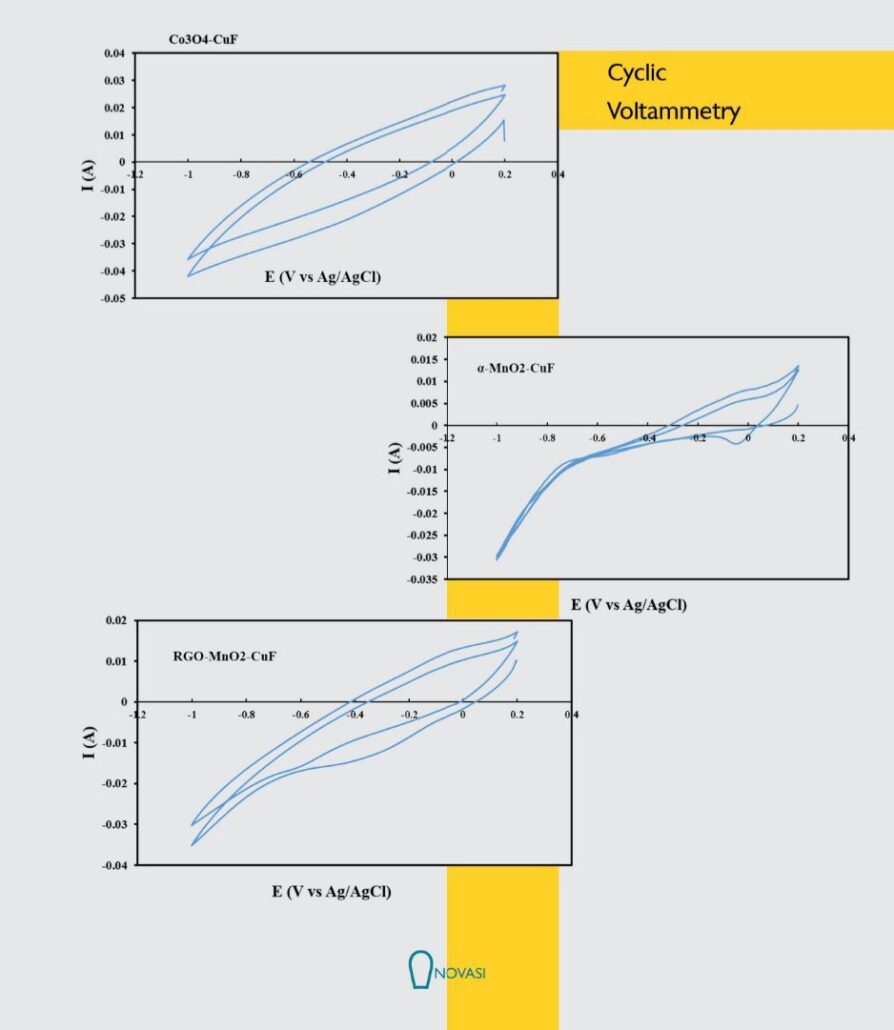
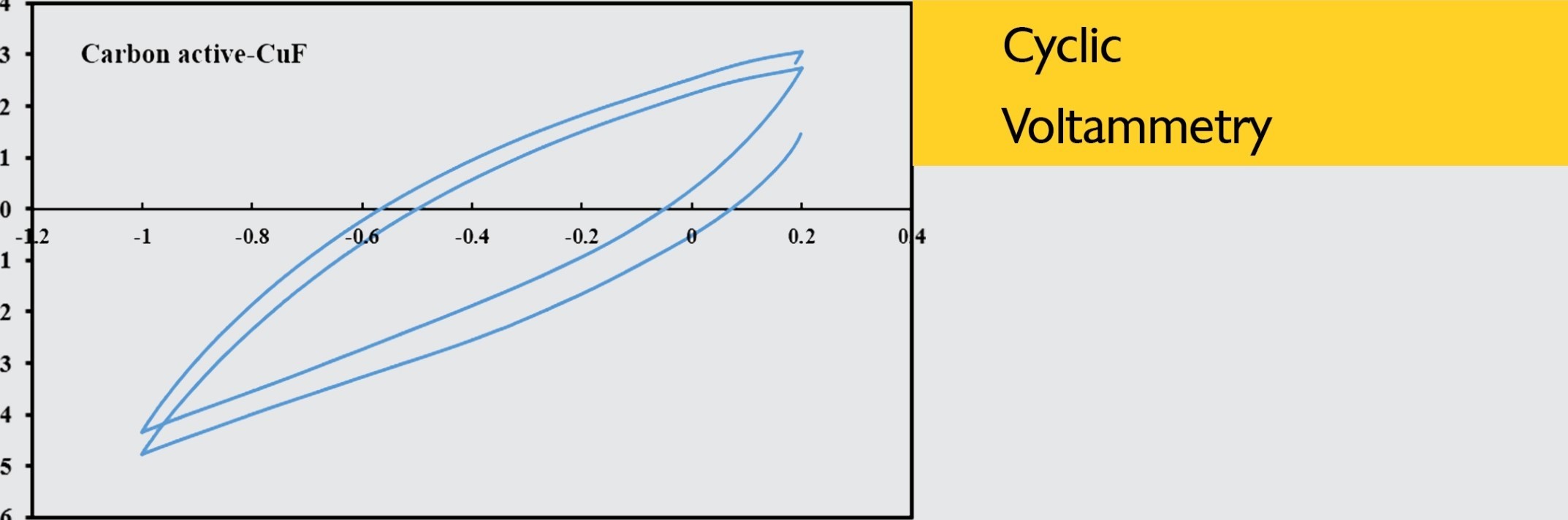
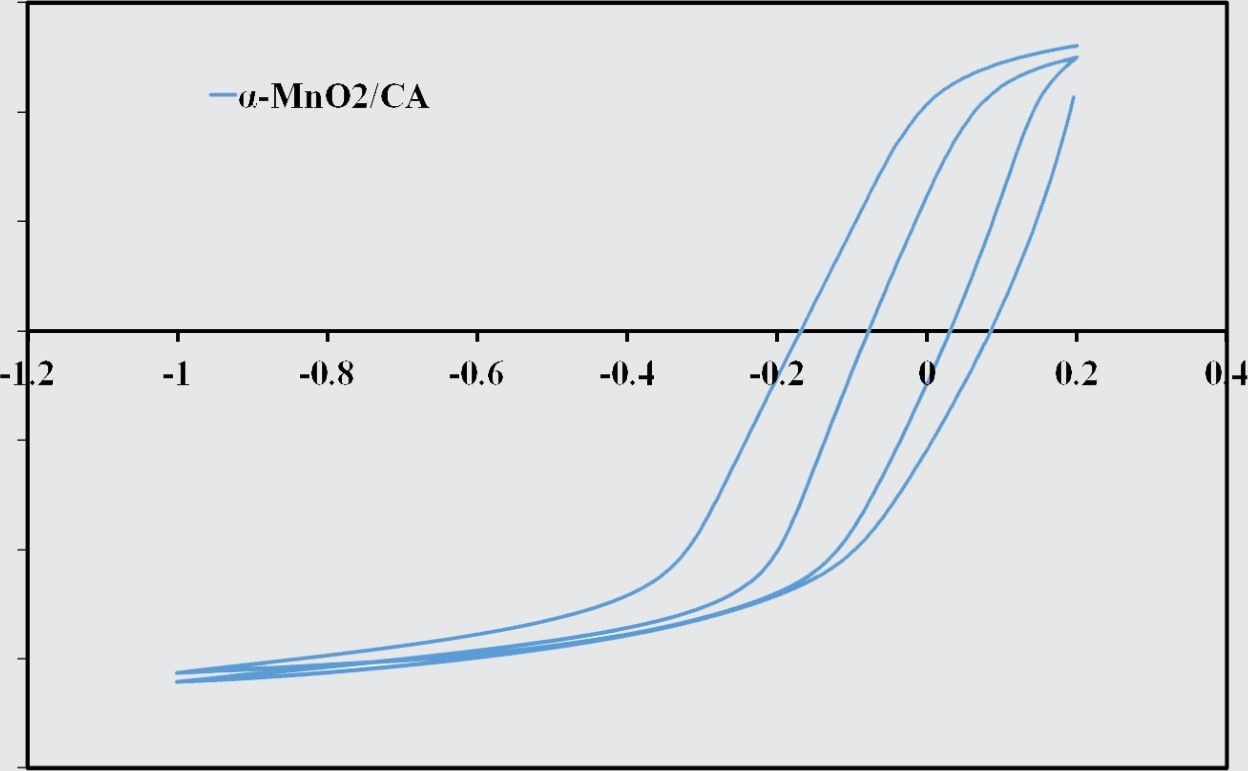
To evaluate the electrocatalytic activity of different catalyst towards oxygen reduction reaction, the cyclic voltammograms (CVs) of five catalysts of Mn02, MnoJCA, CoA-MnoJCA, Mno /RGO and activated carbon were recorded in the presence (O -saturated) of oxygen in 0. I M KOH electrolyte solution at scan rate of 20 mV/s. As can be seen in the cyclic voltammograms (CVs), on the surface of bare carbon cloth electrode in N2-saturated KOH solution, no noticeable oxygen reduction peak is observed while a weak reduction wave is appeared in the 02-purged KOH electrolyte which could be ascribed to the catalytic role of carbon-based substrates for ORR. Among these five catalysts, the RGO a-MnO catalyst shows the most obvious cathodic peak for oxygen reduction, which is in the potential range of 0.2 to -0.6 V
NOVASI